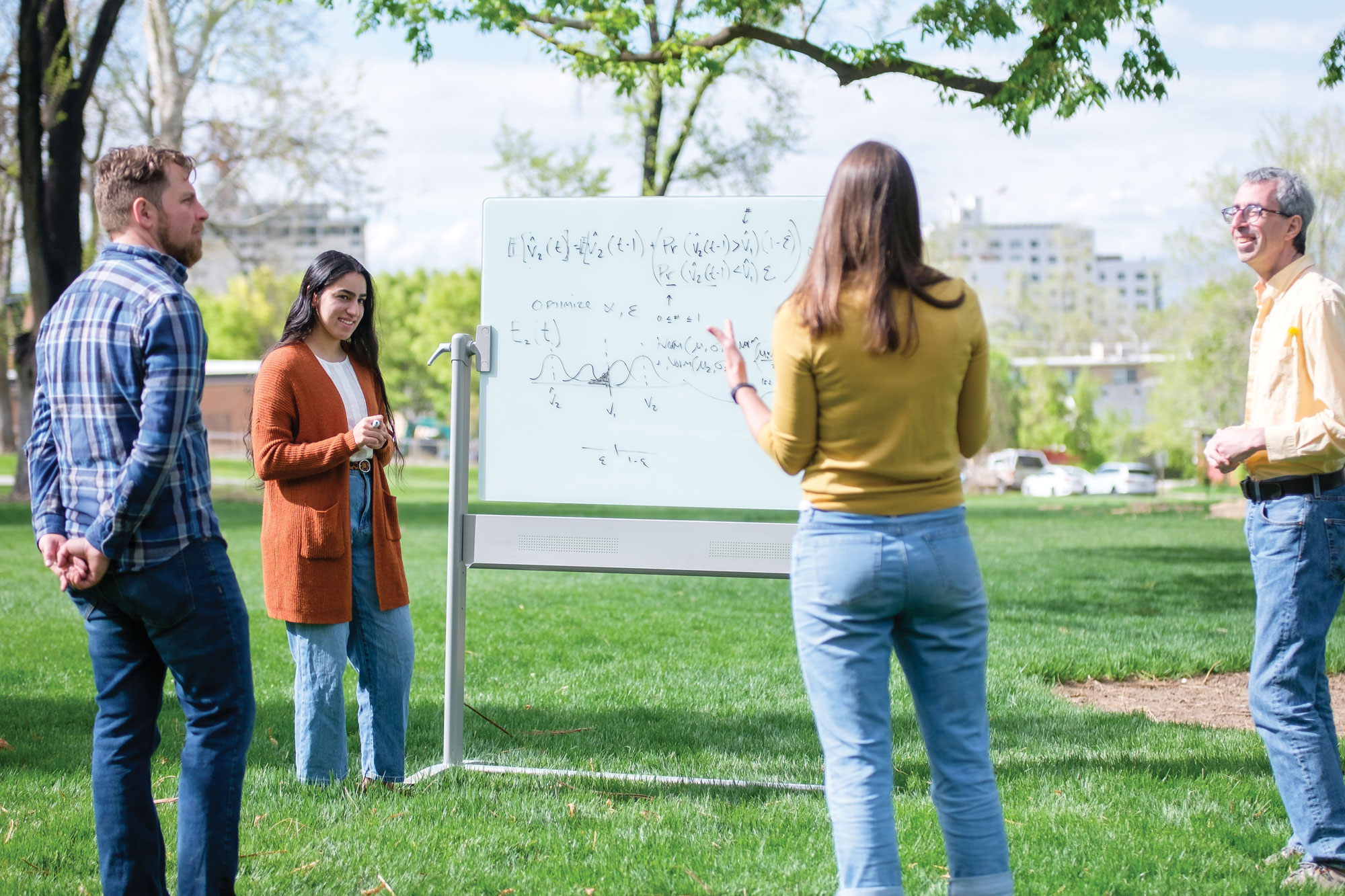
“Cancer cells are often thought of as maverick cells that break the rules and by doing so end up damaging or even killing their host,” says University of Utah professor Fred Adler. “But cancer cells in fact continue to depend on other cells in their environment to survive, particularly under the intense stress we place them under with drug treatment.”
In a recent paper in which Adler is co-author, a team of researchers has determined that while cancer healthcare has been successful in the short term with modern drugs that fight cancer with fewer side effects, long-term success has proven elusive. “Many patients have cancers that recur because some cancer cells evolve resistance to treatment and can resume growth,” he says.
In the study, the team found the surprising result that resistance in breast cancer cells can be shared.
Biting the hand

Fred Adler
In the most common type of breast cancer, treatments almost always target the estrogen pathways that promote growth. And while combinations with modern targeted therapies have proven effective in controlling some cancers, the researchers tested what happens when cells evolve resistance to this combination therapy. “We find, as expected,” continues Adler who has joint appointments in the Department of Mathematics and the School of Biological Sciences where, currently, he is also director, “that these cells are able to grow with combination therapy, unlike their inexperienced drug sensitive cousins. However, we were quite surprised that this resistance is shared with the sensitive cells through over-production of estrogen which enables growth in the presence of treatment. The sensitive cells turn around and ‘bite the hand that feeds them’ by outcompeting the resistance cells.”
Both positive and negative interactions between drug-sensitive and resistant cells can influence the effectiveness of treatment in breast cancer cell populations. Adler and his colleagues have found that the interplay of those interactions in populations that feature differences between cancer cells — both within a single tumor or differences between a primary (original) tumor and a secondary tumor — are especially determinative.
The paper which published June 29th in the journal Nature Communications details how researchers studied interactions between estrogen receptor-positive breast cancer cell lineages that are sensitive and resistant to ribociclib, a kinase inhibitor that blocks the action of an abnormal protein that signals cancer cells to multiply and helps slow or stop the spread of cancer cells.
“In mono- and coculture,” the paper reports, “we find that sensitive cells grow and compete more effectively in the absence of treatment.” During treatment with ribociclib, sensitive cells survive and proliferate better when grown together with resistant cells than when grown in monoculture, termed in ecology as “facilitation,” defined as when one species positively impacts the fitness of another.
With the use of in-vitro model systems, molecular, protein, and genomic analyses showed that resistant cells increase metabolism and production of estradiol — a highly active estrogen metabolite — and increase estrogen signaling in sensitive cells to promote facilitation in co-culture.
Modeling in the Adler Lab
Adler previewed some of these published results last winter at a College of Science-sponsored Science at Lunch event. Eric Slattery, MD, who attended the event, recognized Adler’s skill at synthesizing and simplifying such a complex subject as the fitful, reactive lives of cancer cells. “Cancer viewed as an ecological system is not only novel but helpful in propelling research forward. [For] a physician, basic science research in this field is critical in advancing patient care.”
As a mathematician, Adler, along with his lab collaborators, often use mathematical models focused on viruses, including, recently, the coronavirus that causes Covid-19. But their interests extend to other subject models where ecology, evolution and immunology meet, including, among others, sea ice, the behavior of Southern Right Whales in Argentina, and combat traits of ants. The notion of “facilitation” in the paper — a term stemming from the literature of ecology — is emblematic of the multi-disciplinary, cross-pollination of different sub-fields and approaches in biology and health sciences that are now converging, in this case in the study of cancer cell populations.
Returning to the recently published findings, Adler says of his team, “We used mathematical models to unravel the complexity of these interactions and could then extend them to show how this can be used to more effectively treat cancers.” It turns out, according to the paper, that this kind of modeling “quantifies the strength of competition and facilitation during CDK4/6 inhibition and predicts that blocking facilitation has the potential to control both resistant and sensitive cancer cell populations… .” That potential, when realized, in turn inhibits the emergence of a refractory population during cell cycle therapy.
Gratuitous as a massacre
The authors remind us that breast cancer is the most common cancer worldwide and the second leading cause of cancer death in American women — “gratuitous as a massacre,” to quote the poet Marilyn Hacker, especially when it leads to mastectomies. “The majority (~80%) of these breast tumors,” the authors report, “are estrogen receptor-positive (ER+), and the majority of metastatic patients who die from their cancer have this breast cancer subtype.”
In these tumors, estrogen receptor activity leads to cancer cell proliferation. In order to target both upstream ER and downstream CDK4/6 signaling for cancer control, the combination of CDK inhibitors with endocrine therapy has been used successfully in metastatic ER+ breast cancer, and to a moderate extent in earlier-stage, non-metastatic breast cancer. However, as the research shows, tumors can develop resistance to both single and combination endocrine and cell cycle therapy regimens. Understanding the underlying causes of resistance to endocrine and cell cycle therapies is a critical area of research for this major cancer subtype and cause of death in women.
Clearly, the stakes are high that therapies and even cures for breast cancers are secured. “If we can encourage drug sensitive cells to take advantage of resistant cells,” says Adler, “we can achieve the dual goal of limiting the cancer and of maintaining drug sensitivity to enable long-term cancer control.”
Contributors to the paper titled “Cell facilitation promotes growth and survival under drug pressure in breast cancer,” include the lead co-authors Rena Emond and Jason I. Griffiths along with Rachel S. Sousa (mathematics, University of Utah) and others.
by David Pace
